Draft Proposal
“Biological and digital systems are converging, and could change the way we work, live, and even evolve as a species. More than a technological change, this biodigital convergence may transform the way we understand ourselves and cause us to redefine what we consider human or natural.” - Policy Horizons Canada[1]
Table of Contents
- Introduction
- History of Nanotechnology Regulation
- Current Laws and Regulatory Gaps
- Related Proposals
- Think Tanks and NGOs
- Resources for Further Reading
- Footnotes
Introduction
In the dawn of the biodigital convergence, where nanotechnology seamlessly integrates with human biology through wearables, digital currencies, and data-driven health systems, humanity stands at a crossroads between innovation and intrusion. This fusion promises enhanced connectivity, personalized medicine, and economic efficiency, yet it risks eroding core principles of agency, bodily sovereignty, and privacy without adequate guardrails. This comprehensive proposal seeks to safeguard these rights by tracing the historical evolution of nanotech regulation from early precedents like the U.S. National Nanotechnology Initiative to the current patchwork of laws such as the EU’s REACH framework, highlighting critical gaps in oversight amid rapid technological advancement. By advocating for mechanisms like mandatory opt-out provisions, independent ethics oversight, and international treaties on data sovereignty, this document calls for proactive policies to prevent exploitative abuses, ensuring that individuals retain control over their biodigital selves in an increasingly merged world.[2][3][4]
For further understanding, watch this short YouTube video explaining biodigital convergence:
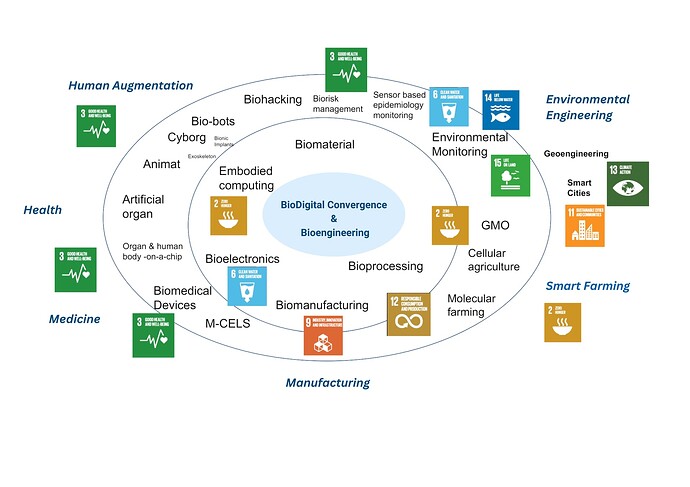
To visually illustrate the concept of biodigital convergence, here’s a relevant image from the International Electro Technical Commission depicting the integration of biology and digital tech:
History of Nanotechnology Regulation
The regulation of nanotechnology has evolved sporadically since its conceptual inception, often lagging behind scientific advancements and focusing primarily on environmental and occupational health risks rather than comprehensive human rights safeguards in the context of biodigital convergence. This section outlines key historical milestones, precedents, and laws, highlighting the lack of robust frameworks for protecting bodily sovereignty, agency, and data rights as nanotech integrates with biology and digital systems.[5][6][7]
Early Conceptual Foundations (1950s-1990s)
Nanotechnology’s history begins with physicist Richard Feynman’s 1959 lecture, “There’s Plenty of Room at the Bottom,” which envisioned manipulating matter at the atomic scale.[8] The term “nanotechnology” was coined by Norio Taniguchi in 1974, but it gained prominence through K. Eric Drexler’s 1986 book, “Engines of Creation,” which discussed molecular assemblers and raised early ethical concerns about self-replicating machines.[8] Breakthroughs like the 1981 scanning tunneling microscope (STM) and 1985 discovery of fullerenes laid technological groundwork, but regulatory discussions were minimal, with no specific laws addressing human health implications until the late 1990s.[8]
Emergence of Convergence Concepts (1990s-2000s)
The 1998 book “Consilience” by E.O. Wilson proposed integrating scientific disciplines, setting the stage for convergence ideas like NBIC (Nano-Bio-Info-Cogno) in 2002-2005, as explored in the NSF report “Converging Technologies for Improving Human Performance.”[9][10][11] This report emphasized accelerating human performance through tech integration but lacked emphasis on rights safeguards.[11] In 2000, the U.S. established the National Nanotechnology Initiative (NNI) under President Clinton, focusing on R&D with over $1 billion in funding by 2003, but it prioritized innovation over regulation, creating gaps in health oversight.[8]
Initial Regulatory Efforts and Precedents (2000s)
By 2003, the UK Better Regulation Commission recommended public engagement on nanotech risks, emphasizing transparency.[12] The 2004 UK Royal Society report identified nanoparticle toxicity risks, advocating for precautionary regulation and treating nanomaterials as hazardous.[8][12] In the U.S., California passed AB 289 in 2006, requiring manufacturers to provide health impact data.[12] Berkeley became the first U.S. city to regulate nanotech in 2006, while the Bush administration in 2007 deemed no special regulations necessary, drawing criticism for exposing consumers to untested risks.[12] The 2008 U.S. National Research Council called for more oversight, and California’s DTSC initiated “call-ins” for nanomaterial data in 2009-2010.[12]
In the EU, the REACH regulation (2007) began addressing nanomaterials, requiring health data for carbon products like nanotubes.[12] National registries emerged in France, Belgium, Sweden, and Denmark by the 2010s, and the European Union Observatory for Nanomaterials (EUON) was established to collect safety information.[12]
Biodigital Convergence and Modern Gaps (2010s-Present)
The 2015 MANBRIC framework expanded convergence concepts, leading to the IEC’s identification of biodigital convergence (BDC) in 2020 as the merger of nano, bio, info, and cogno technologies.[11][13] Policy Horizons Canada’s 2020 and 2024 reports highlighted BDC’s transformative potential but warned of human rights risks, such as DNA profiling in immigration leading to violations, and called for ethical frameworks.[1][3][14] The UCL 2024 report on BDC standards emphasized integrating ethics, human dignity, and rights into regulations, recommending adaptive governance like PAGIT to address gaps in data privacy and bodily autonomy.[4][11] Globally, there remains no unified international regulation, with NGOs like Friends of the Earth advocating for precautionary principles, mandatory labeling, and manufacturer liability since 2008.[12][15] Ethical concerns include nanoparticle toxicity causing DNA damage and environmental harm, with inadequate funding for health research.[8][12] In biodigital contexts, safeguards like informed consent, opt-out mechanisms, and equity in access are largely absent, exacerbating risks to agency and sovereignty.[16][17][11]
This history reveals a reactive, fragmented approach, with precedents like REACH and NNI focusing on R&D and safety but neglecting broader rights in convergence scenarios.
View the YouTube video, “From Atoms to Innovations: The Power of Nanotechnology for a Better Future!” to learn more about how developed nanotech has become:
Current Laws and Regulatory Gaps
As of 2025, laws regulating nanotechnology in relation to human beings remain fragmented and primarily focused on environmental health and safety rather than comprehensive protections for bodily sovereignty, agency, and opt-out rights in the biodigital era. This section examines key current frameworks in the United States, European Union, and globally, including those intersecting with wearables, digital data, and currency. It highlights persistent gaps that expose individuals to risks from surveillance, data exploitation, and unconsented integration of nanotech with biology.[9][18][19][20]
United States Frameworks
In the US, the National Nanotechnology Initiative (NNI) continues to coordinate federal R&D, with an updated Environmental, Health, and Safety (EHS) Strategy released in 2025 to address emerging nanotech challenges.[6][21] The Environmental Protection Agency (EPA) regulates nanoscale materials under the Toxic Substances Control Act (TSCA), requiring pre-manufacture notices for new nanomaterials and issuing consent orders or Significant New Use Rules (SNURs) for limited production.[22][23] The Food and Drug Administration (FDA) oversees nanomedicine through programs assessing nanomaterials’ interactions with biological systems, as codified in 21 U.S. Code § 399e, which mandates reviews of scientific data on nanomaterials in food, drugs, and cosmetics.[24][25] For digital health, the 2025 Digital Health Laws and Regulations Report outlines rules on data use and sharing in wearables, but lacks specific opt-out mechanisms for biodigital integrations.[9][26] On digital currency, the Anti-CBDC Surveillance State Act, passed in 2025, prohibits the Federal Reserve from issuing central bank digital currencies (CBDCs) to prevent surveillance and protect financial privacy.[27][28][29] The GENIUS Act establishes a framework for stablecoins, emphasizing privacy, while FDIC guidance clarifies banks’ engagement in crypto activities without mandating robust anti-surveillance measures.[30][31]
European Union Frameworks
The EU lacks dedicated regulations for nanomaterials but applies existing laws like REACH, which requires safety data for substances including nanotubes.[32][33] The European Union Observatory for Nanomaterials (EUON) collects and disseminates safety information, with a clarified definition of nanomaterials updated in 2022 and ongoing in 2025.[33][34] For wearables and digital data, the European Health Data Space (EHDS) Regulation, effective from 2025, facilitates health data sharing but raises concerns over public interest uses without strong opt-out provisions.[35] The EU Data Act, applicable from September 2025, imposes data sharing obligations on connected products like wearables, impacting medical devices but not fully addressing biodigital privacy risks.[36][37]
Global and International Frameworks
Internationally, there is no unified treaty on nanotech or biodigital convergence, leading to divergent approaches. Countries like Canada, China, Japan, and Australia have varying safety frameworks, with the US and EU influencing trading partners like Israel through export rules.[38][39] The World Economic Forum’s Global Risks Report 2025 identifies nanotech and AI convergence as risks without adequate governance.[40]
Key Regulatory Gaps
Despite these frameworks, significant gaps persist. Regulations often treat nanomaterials under general chemical laws, ignoring unique biodigital risks like nanoparticle toxicity in human tissues or surveillance via integrated wearables.[41][13][20] Opt-out mechanisms are rare, with privacy laws like the US’s proposed Anti-CBDC Act addressing surveillance in finance but not extending to nanotech-enhanced data collection.[14][42] Global inconsistencies exacerbate vulnerabilities to exploitative uses, underscoring the need for unified safeguards on bodily sovereignty and agency.[19][1][43]
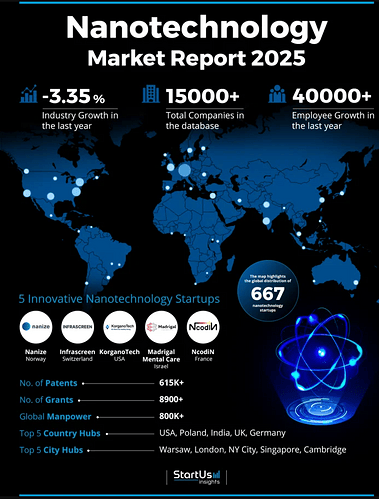
For a visual overview of global nanotech market, consider this map:
Related Proposals
-
Digital Freedom Policy Framework
Outlines a U.S.-centric vision for digital sovereignty, privacy, and innovation, emphasizing civilian-based cyber defense and a Digital Bill of Rights. -
Digital Privacy Act of 202X
Proposes comprehensive digital privacy laws, including consumer rights to transparency, consent, and data deletion, with restrictions on AI and government access. -
Prohibit Governmental Purchase of User Data and Private Information from Third Parties
Advocates banning government purchase of personal data to protect privacy and prevent surveillance abuses.
(Will Add More Here)
Think Tanks and NGOs
Below is a compiled, unique list of think tanks, NGOs, and advocacy groups focused on digital rights, privacy, human rights in technology, biodigital convergence, nanotechnology regulation, and related areas. This draws from various sources for comprehensive coverage, prioritizing relevance to the proposal’s themes. Descriptions and links are included where available.
Click to See 25 Relevant Organizations
-
Access Now
Defends digital rights of at-risk users globally, organizing events like RightsCon on human rights and tech. -
AI Now Institute
Researches social implications of AI, including rights, bias, and critical infrastructure. -
Algorithmic Justice League
Raises awareness of AI harms, advocates for impacted communities, and mitigates biases. -
American Civil Liberties Union (ACLU)
Defends constitutional rights, including privacy in technology and surveillance issues. -
Amnesty Tech (Amnesty International)
Challenges surveillance, AI ethics, and censorship through advocacy and research. -
Aspen Digital (The Aspen Institute)
Promotes responsible stewardship of tech for equity and justice. -
Center for AI and Digital Policy
Promotes fair AI policies grounded in human rights and democracy. -
Center for Democracy and Technology (CDT)
Shapes tech policy to protect privacy, free expression, and individual rights. -
Center for Humane Technology
Reimagines digital infrastructure for well-being and democracy. -
Center for Strategic and International Studies (CSIS)
Advances policy on cybersecurity, tech, and global challenges. -
Cities Coalition for Digital Rights
Promotes digital rights in urban contexts to resolve tech challenges. -
Digital Health and Rights Project (Fondation Botnar)
Focuses on human rights in digital health, involving social scientists and advocates. -
Digital Rights Foundation
Advocacy NGO on ICTs for human rights, democracy, and digital governance. -
Electronic Frontier Foundation (EFF)
Defends civil liberties in the digital world, focusing on privacy and innovation. -
European Digital Rights (EDRi)
Network defending online rights and freedoms against surveillance. -
Future of Humanity Institute (FHI)
Researches long-term tech impacts, including AI and biotechnology risks. -
Global Network Initiative
Protects human rights in the digital age through principles and accountability. -
Harvard Kennedy School - Technology & Human Rights
Explores tech’s impact on human rights and future protections. -
Montreal AI Ethics Institute
Democratizes AI ethics literacy for citizen action. -
New America
Addresses tech-driven social changes through public interest technology. -
Oxford Internet Institute
Studies social science of the internet, including behavior and policy. -
Pew Research Center - Internet & Technology
Conducts nonpartisan research on tech trends and policy issues. -
Ranking Digital Rights
Ranks companies on commitments to user privacy and expression. -
Tactical Tech
Partners on media, digital literacy, and rights in tech. -
World Economic Forum’s Centre for the Fourth Industrial Revolution
Shapes policies for emerging tech like AI and biotech convergence.
Resources for Further Reading
In addition to the proposals listed in the related proposals section, here’s an expanded list of key resources on digital bills of rights, biodigital convergence, and nanotech regulation:
-
Bio-digital Convergence Standardization Opportunities (IEC Report)
Discusses convergence of nanotechnology, biotech, and IT. -
Future Standards for Bio-digital Convergence (UCL Report)
Proposes standards emphasizing human dignity and ethics. -
Regulatory Landscape of Nanotechnology and Nanoplastics
Overview of global regulatory frameworks. -
Why Converging Technologies Need Converging International Regulation
Ethical and policy challenges of tech convergence. -
Converging Technologies for Improving Human Performance (NSF Report)
Early exploration of nano-bio-info-cogno convergence. -
Ethical and Legal Challenges in Nanomedical Innovations
Scoping review of nanomedicine ethics. -
The Postdigital-Biodigital Revolution
Discusses rights in a biodigital context. -
A Regulatory Framework for Nanotechnology
U.S. perspective on nanotech proliferation and regulation. -
Tiny Tech, Big Impact: Nanotechnology and SDGs
Role of nanotech in sustainable development. -
Securing the Promise of Nanotechnologies (LSE Report)
Transatlantic regulatory cooperation on nanotech.
Footnotes
[1] Policy Horizons Canada, “The Biodigital Convergence”
[2] Blueprint for an AI Bill of Rights
[3] Exploring Biodigital Convergence
[4] Future Standards for Bio-digital Convergence
[5] Florida Digital Bill of Rights
[6] Digital Equity Bill of Rights
[7] Establishing a Digital Bill of Rights
[8] Regulatory Landscape of Nanotechnology and Nanoplastics
[9] Converging Technologies for Improving Human Performance
[10] Internet Bill of Rights
[11] Why Converging Technologies Need Converging International Regulation
[12] Securing the Promise of Nanotechnologies
[13] Bio-digital Convergence Standardization Opportunities
[14] Ethical and Legal Challenges in Nanomedical Innovations
[15] Tiny Tech, Big Impact: Nanotechnology and SDGs
[16] Global Network Initiative
[17] Harvard Kennedy School - Technology & Human Rights
[18] A Regulatory Framework for Nanotechnology
[19] Legislation to Ban Central Bank Digital Currencies
[20] Creating a ‘Digital Bill of Rights’
[21] National Nanotechnology Initiative EHS Strategy 2025
[22] EPA Nanotechnology Regulation
[23] TSCA Section 8(a) Chemical Data Reporting
[24] FDA Nanotechnology Task Force Report
[25] 21 U.S. Code § 399e
[26] Digital Health Laws and Regulations Report 2025
[27] CBDC Anti-Surveillance State Act
[28] House Passes CBDC Anti-Surveillance Act
[29] Emmer Whip Check on CBDC
[30] GENIUS Act Stablecoin Framework
[31] FDIC Crypto Guidance
[32] REACH Regulation Nanomaterials
[33] European Union Observatory for Nanomaterials
[34] EU Nanomaterials Definition Update 2022
[35] European Health Data Space Regulation
[36] EU Data Act
[37] EU Data Act and Medical Devices
[38] Global Nanotechnology Regulation Overview
[39] Nanotechnology Regulation in Israel
[40] World Economic Forum Global Risks Report 2025
[41] Ethical and Legal Challenges in Nanomedicine
[42] Nanotechnology and Human Rights
[43] Should a “Digital Bill of Rights” be adopted?